PRODUCTS & RD
The new generation of small molecular anti-cancer drugs, which are under laboratory and animal experiments, are effective against multiple cancers. The phase II clinical trial is estimated to recruit patients in Australia in 2019Q2.
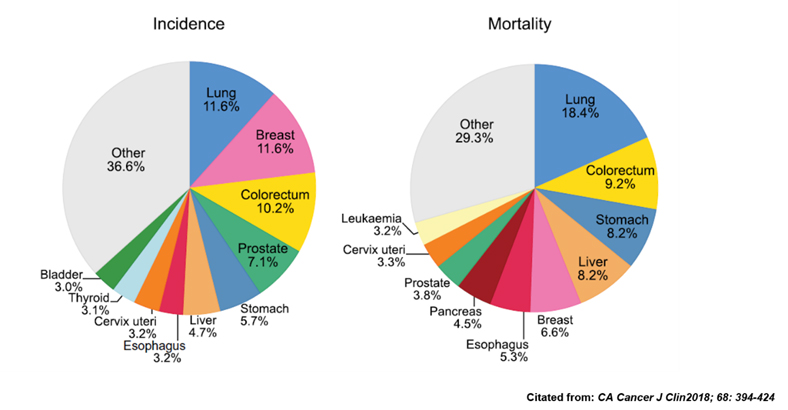
According to the World Health Organization (WHO) and Global Cancer Statistics 2018, cancer is the second leading cause of death in the world. Furthermore, 18 million new cases of cancer patients were diagnosed in 2018 and 9.6 million died of cancers especially in Lung (18.4%), colorectal (9.2%), stomach (8.2) and liver (8.2%) cancer. Moreover, according the data from Ministry of Health and Welfare in Taiwan, cancer is the first cause of death in Taiwan in 2017 and the top three indications are lung, liver and colorectal cancer.
Cancer patients undergo targeted drug therapy nowadays are still facing the facts that some drug-resistant cancer cells cannot be completely eradicated, leading to the recurrence of the disease, and patients sometimes may suffer serious side effect from the treatment. A targeted drug, Sorafenib has been approved for advanced renal cell carcinoma and liver cancer treatment, is a tyrosine kinase inhibitor which inhibits Raf in cancer cell and block the RAF / MEK / ERK signaling pathway. It can also inhibit tyrosine protein kinases, such as VEGFR, PDGFR, hormone receptor (c-KIT cytokine receptor) and several others to inhibit tumor growth. However, its large scale of inhibition mechanism will lead to patients suffer from high blood pressure, myocardial infarction, heart failure, hand-foot syndrome, bleeding, diarrhea and other side effects, moreover, the possibility of disease recurrence.
MG010, a newly synthesized compound developed by Metagone, is a C-Raf inhibitor, which demonstrates inhibition effect on mitochondrial C-Raf and the phosphorylated Death-Associated Protein Kinase (DAPK) complex in cancer cell together with another C-Raf inhibitor-Sorafenib. This MG-D-1609 will cause mitochondria dysfunction, therefore, cut off energy supply and leading to cancer cell death. The MG-D-1609 can accurately identify and kill specific cancer cells, lower down the possibility of side effects and drug resistance. The in vitro and in vivo results have showed the great efficacy of 1609 in lung, colorectal, kidney and liver cancer cell lines. Since the urgent need of this disease but lack of better options for patients suffered from above cancers. MG-D-1609 may be a breakthrough drug and expected to bring hope for patients with cancer patients. The MG-D-1609 is estimated to execute phase II clinical trial to prove the efficacy on lung, colorectal, liver and kidney cancer patients in Australia in 2019Q2.
According to Global Cancer Statistics 2018, lung, colorectal, liver and kidney cancer patients were about 4.4 million which is about 24% of total cancer patients. This issue (cancer) is regarded as an important international health crisis. With aging, poor living environment and eating habits change problems, the demand on drugs treating. It is estimated that the drug will reach more than 27.2 billion U.S. dollars by 2024 in the global market.