News Center

2022 12 Metagone BREAKING NEWS
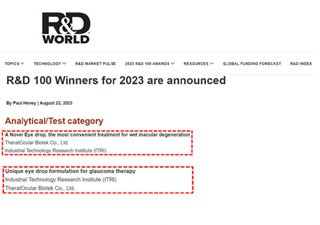
 ITRI Special Issue ----Interview with Dr. Sean Chen, chairman of TheratOcular Biotek
ITRI Special Issue ----Interview with Dr. Sean Chen, chairman of TheratOcular Biotek

2022 12 Metagone BREAKING NEWS
 New blue ocean for ophthalmology drugs; Taiwanese manufacturers are actively improving innovative technologies
New blue ocean for ophthalmology drugs; Taiwanese manufacturers are actively improving innovative technologies

2022 11/24 Metagone BREAKING NEWS
 New blue ocean for ophthalmology drugs; Taiwanese manufacturers are actively improving innovative technologies
New blue ocean for ophthalmology drugs; Taiwanese manufacturers are actively improving innovative technologies

2022 11/24 Metagone BREAKING NEWS
 Old formula New amazing effect, TheratOcular Biotek makes it easy to treat eye diseases - Executive VP Simon Huang Interview
Old formula New amazing effect, TheratOcular Biotek makes it easy to treat eye diseases - Executive VP Simon Huang Interview

2022 11/18 Metagone BREAKING NEWS
 63thAnnual Meeting of Ophthalmology & 15th Asia Pacific Vitreoretinal Society (APVRS) Congress
63thAnnual Meeting of Ophthalmology & 15th Asia Pacific Vitreoretinal Society (APVRS) Congress

2022 08/15 Metagone BREAKING NEWS
 The phase II clinical trial of TO-O-1002 approved by the Central Research Ethics Committee (CREC) in Thailand on 11 Aug 2022.
The phase II clinical trial of TO-O-1002 approved by the Central Research Ethics Committee (CREC) in Thailand on 11 Aug 2022.

2022 08/09 Metagone BREAKING NEWS
 The phase I/II clinical trials of TO-O-1001 approved by the Australian Human Research Ethics Committee (HREC) on 4 Aug 2022.
The phase I/II clinical trials of TO-O-1001 approved by the Australian Human Research Ethics Committee (HREC) on 4 Aug 2022.

2022 08 Metagone BREAKING NEWS
 The company Chair, Dr. Sean Chen, and the SAB Chair, Dr. Lu Da-Wen, get together with all the honorary members of the Company Scientific Advisory Board (SAB) in a banquet.
The company Chair, Dr. Sean Chen, and the SAB Chair, Dr. Lu Da-Wen, get together with all the honorary members of the Company Scientific Advisory Board (SAB) in a banquet.

2022 07 Metagone BREAKING NEWS
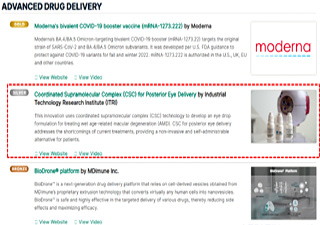
 TheratOcular Team meet up with its RD/BD partner, the ITRI-BEL team, at the 2022 Asia Biotech Exhibition.
TheratOcular Team meet up with its RD/BD partner, the ITRI-BEL team, at the 2022 Asia Biotech Exhibition.

2020 12 Metagone BREAKING NEWS
 Metagone Biotech drug of lung disease has won 17th WE innovators award
Metagone Biotech drug of lung disease has won 17th WE innovators award

2018 11 Metagone BREAKING NEWS
 TFDA approves Clinical Trial Application for MG-S-2525 Phase 1 Studies
TFDA approves Clinical Trial Application for MG-S-2525 Phase 1 Studies

2018 11 Metagone BREAKING NEWS
 Participated in the 22nd Beijing Biomedical Industry Development Forum to exchange and promote more cooperation.
Participated in the 22nd Beijing Biomedical Industry Development Forum to exchange and promote more cooperation.

2018 07 Metagone BREAKING NEWS
 信力生技針對肺纖維化疾病(IPF )的MG-S-2525(First in class)小分子新藥通過美國食品藥物管理局(FDA)許可開始進行一期人體臨床試驗
信力生技針對肺纖維化疾病(IPF )的MG-S-2525(First in class)小分子新藥通過美國食品藥物管理局(FDA)許可開始進行一期人體臨床試驗

2018 05 Metagone BREAKING NEWS
 TFDA approves Clinical Trial Application for MG-D-1509 Phase 1/2a Studies.
TFDA approves Clinical Trial Application for MG-D-1509 Phase 1/2a Studies.

2017 11 Metagone BREAKING NEWS
 US FDA approves Clinical Trial Application for MG-D-1509 Phase 1/2a Studies.
US FDA approves Clinical Trial Application for MG-D-1509 Phase 1/2a Studies.

2016 12 IBMI
 Precision Medicine with Novel Raf Inhibitor Combination Therapy and Companion Predictive Biomarker
Precision Medicine with Novel Raf Inhibitor Combination Therapy and Companion Predictive Biomarker

2015 09
Cancer Research Professional journals reported

2015 08
Metagone Biotech Inc. and National Defense Medical Center have signed a mutual technology transfer license agreement